In vivo infection inhibition in rat osteomyelitis model with sol gel coated pins containing vancomycin
Peri-prosthetic infection remains a serious complication of joint replacement surgery. We demonstrated that a vancomycin-containing sol-gel film on Ti alloy rods can successfully treat bacterial infections in an animal model. The vancomycin-containing sol-gel films exhibited predictable release kinetics, while significantly inhibiting S. aureus adhesion. When evaluated in a rat osteomyelitis model, microbiological analysis indicated that the vancomycin-containing sol-gel film caused a profound decrease in S. aureus number. Radiologically, while the control side showed extensive bone degradation, including abscesses and an extensive periosteal reaction, rods coated with the vancomycin-containing sol-gel film resulted in minimal signs of infection. µCT analysis confirmed the radiological results, while demonstrating that the vancomycin-containing sol-gel film significantly protected dense bone from resorption and minimized remodeling. These results clearly demonstrate that this novel thin sol-gel technology can be used for the targeted delivery of antibiotics for the treatment of periprosthetic as well as other bone infections.
© J Orthop Res 27:701–709, 2009
Vancomycin-containing
sol-gel coated Ti rods exhibit controlled antibiotic release, stable coverage,
and effective microbicidal activity. (A) Vancomycin release into PBS was
measured as described and expressed as a function of applied layers (l) and concentration
(%). Note the increase in vancomycin release as the number of layers with 10%
vancomycin in each increase from three to five. Additional increase in the
release from 5l-coating is observed with increase of vancomycin concentration
in the last two layers from 10 to 20%. By day 6 of immersion, 85, 78, and 70%
of the original load was released from the 3l-10V, 5l-10V, and 5l-10V+20V
coatings, respectively. (B) Bacterial growth on coated Ti rods challenged with S.
aureus. Note the SGV coating caused a nearly fourfold decrease in bacteria. (C)
SEM of the sol-gel surface after incubation with bacteria. Values shown
represent mean and SEM. *p<0.05.
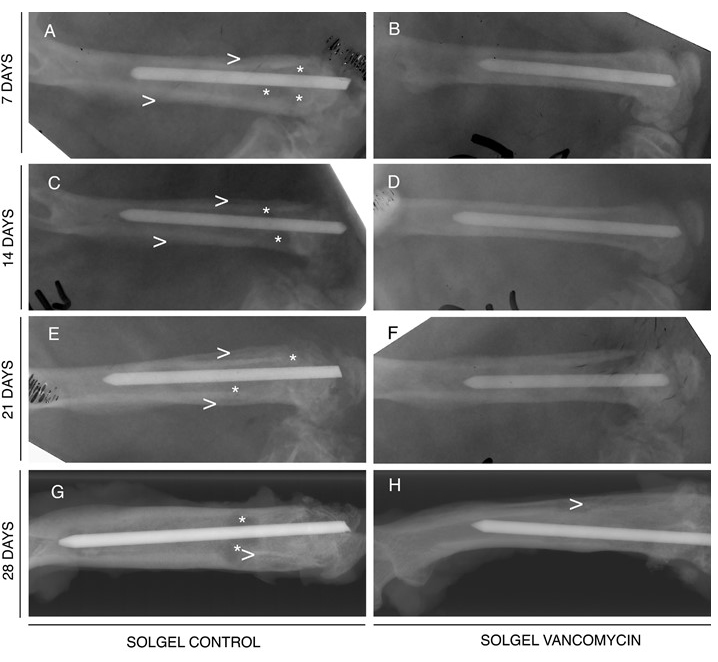
Radiographic analysis indicates
that vancomycin- containing sol-gel coated Ti rods prevent osteomyelitis. Vancomycin-containing
sol-gel (SGV) (right) and control sol-gel (SG) (left) coated Ti rods were implanted
into infected rat femora. The femora were harvested at 7 (A,B), 14 (C,D), 21
(E,F), or 28 (G,H) days. Note that while the SG rods show unmistakable signs of
bone infection (periosteal elevation, enlargement of the medullary canal [>],
as well as focal cysts [*]), there are minimal changes in the bones treated
with SGV rods.
The incidence of methicilin-resistant Staphylococcus
aureus (MRSA) infection has
significantly increased. Generally, the success of this bacterium as a pathogen
is attributed to its ability to adhere to surfaces and remain there, under the
protection of an extracellular matrix known as biofilm. To combat MRSA with regular
doses of vancomycin, efforts are continuously underway to increase its
effectiveness. A promising technique is to use combinational therapeutics. In vitro experiments showed that farnesol
can be used as an adjuvant with conventional antibiotics. Farnesol is a natural
sesquiterpenoid and quorum-sensing molecule. The biggest obstacle to using this
concept is that farnesol is highly water insoluble. This compromises its
bioavailability if it were to be used along with vancomycin at the site of
infection when the treatment needs to be administered in vivo.
Herein we designed an efficient therapeutic strategy for the simultaneous
delivery of both antibiotic and adjuvant in order to treat MRSA infections. We
demonstrate that sufficient quantities of both vancomycin and farnesol can be
incorporated into sol–gel silica applied as thin films on an implant surface.
The incorporation of the hydrophobic farnesol does not affect the stability of
the thin films and neither does it affect the controlled release of vancomycin.
The data demonstarte the potent adjuvant effect of farnesol on vamcomycin in inhibiting MRSA infection
In vitro experiments show the complete
inhibition (106 fold
reduction in growth compared to control) of methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) when the ratio of
vancomycin to farnesol in the sol–gel silica films is optimized. The local
delivery of antibiotics minimizes the need for systemic antibiotics. The
incorporation of vancomycin and farnesol into thin sol–gel films represents a
new treatment paradigm for the topical delivery of antibiotics with adjuvant.
The potential clinical benefits are significant and include avoiding the need
for revision surgery, preventing surgical site infection and controlling
healthcare costs.
© Biomaterials
35:509-517, 2014
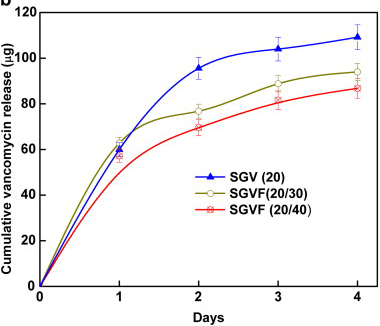
In vitro cumulative
release of vancomycin from the sol–gel coating on K-wires containing:
20 wt% vancomycin (SGV(20)), 20 wt% vancomycin and 30 wt%
farnesol (SGVF(20,30)), 20 wt% vancomycin and 40 wt% farnesol
(SGVF(20,40)).
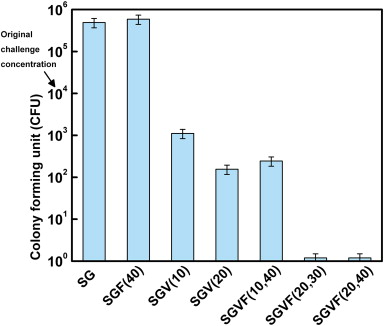
The bactericidal effects
of thin sol–gel films on K-wires containing vancomycin and farnesol alone as
well as in combination against MRSA growth. K-wires were coated with thin
sol–gel films were challenged by a 104 CFU/mL solution of MRSA.
External
fracture fixation in rabbit model with external fixator pins coated with sol
gel films containing the bactericidal molecule triclosan
Risk of infection is
considerable in open fractures, especially when fracture fixation devices are
used to stabilize the fractured bones. Overall deep infection rates of 16.2%
have been reported. The infection rate is even greater, up to 32.2%, with
external fixation of femoral fractures. The use of percutaneous implants for
certain clinical applications, such as percutaneous implants for external
fracture fixation, still represents a challenge today.
Currently, bone infections are very difficult to treat. Very potent antibiotics are needed, which creates the risk of irreversible damage to other organs, when the antibiotics are administered systemically. As such, controlled, local release is being pursued, but no such treatments are in clinical use. Herein, the use of bactericidal micron-thin sol–gel films on metallic fracture fixation pins is reported. The data demonstrates that triclosan (2,4,4′-trichloro-2′-hydroxydiphenylether), an antimicrobial agent, can be successfully incorporated into micron-thin sol–gel films deposited on percutaneous pins. The sol–gel films continuously release triclosan in vitro for durations exceeding 8 weeks (longest measured time point). The bactericidal effect of the micron-thin sol–gel films follows from both in vitro and in vivo studies. Inserting percutaneous pins in distal rabbit tibiae, there were no signs of infection around implants coated with a micron-thin sol–gel/triclosan film. Healing had progressed normally, bone tissue growth was normal and there was no epithelial downgrowth. This result was in contrast with the results in rabbits that received control, uncoated percutaneous pins, in which abundant signs of infection and epithelial downgrowth were observed. Thus, well-adherent, micron-thin sol–gel films laden with a bactericidal molecule successfully prevented pin tract infection.
© Biomaterials 62:95-105,
2015
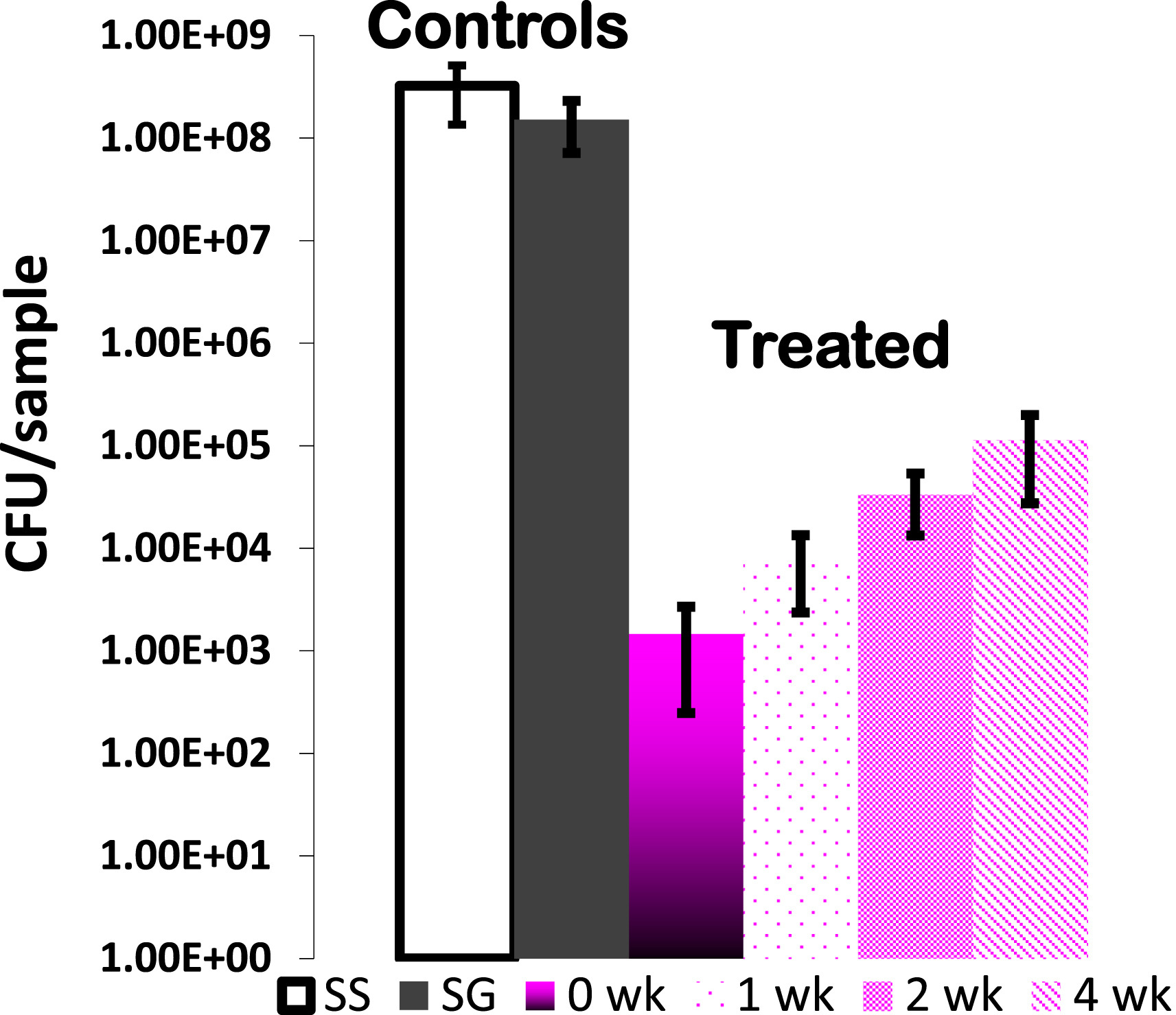
The number of S. aureus in
bacterial cultures after incubation in the presence of pins that were uncoated
(SS), sol–gel coated without triclosan (SG), and sol–gel coated with 20%
triclosan (SGT) after partial elution up to 0, 1, 2 and 4 weeks.
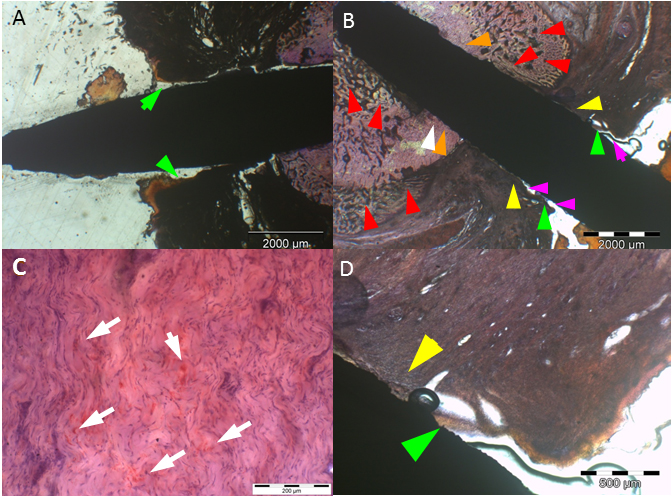
a: Uncoated (SS) implant
at 4 weeks with extensive epithelial downgrowth (green arrows), i.e. pocket
formation. b: Uncoated (SS) implant at 4 weeks with bacterial colonization and
infiltration of S. aureus in the subcutaneous
tissue (white arrows) (deacrylated sawed section immunostained for S. aureus).
c: Histomicrograph of resin embedded section of an uncoated (SS) implant
stained with hematoxylin after deacrylation. 4 weeks after implantation of a SS
implant extensive pocket (black & green arrows) and eschar formation
(arrowheads) is present. In addition, an inflammatory round cell infiltrate is
visible (yellow arrows) (Undecalcified sawed section;
bar = 20 μm). d: Histomicrograph of resin embedded uncoated (SS)
implant section stained enzyme-histochemically for TRAP (Tartrate resistant
alkaline phosphatase) after deacrylation. 4 weeks after implantation, TRAP
positive osteoclasts are visible (black arrows and encircled). This is
indicative of bone resorption at the bone implant interface (Undecalcified
sawed section counterstained with hematoxylin; bar = 20 μm).
(For interpretation of the references to color in this figure legend, the
reader is referred to the web version of this article.)
Local, Controlled Delivery of Local Anesthetics In Vivo from Polymer - Xerogel Composites
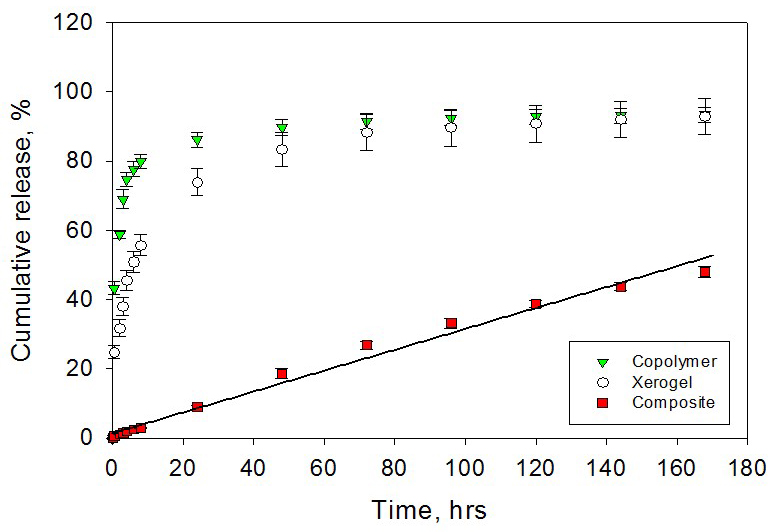
Polymer-xerogel composite
materials have been introduced to better optimize local anesthetics release
kinetics for the pain management. In a previous study, it was shown that by
adjusting various compositional and nano-structural properties of both
inorganic xerogels and polymers, zero-order release kinetics over 7 days
can be achieved in vitro. In this study, in vitro release
properties are confirmed in vivo using a model that tests for
actual functionality of the released local anesthetics.
Composite materials made
with tyrosine-polyethylene glycol(PEG)-derived poly(ether carbonate) copolymers
and silica-based sol–gel (xerogel) were synthesized. The in vivo release
from the composite controlled release materials was demonstrated by local
anesthetics delivery in a rat incisional pain model.
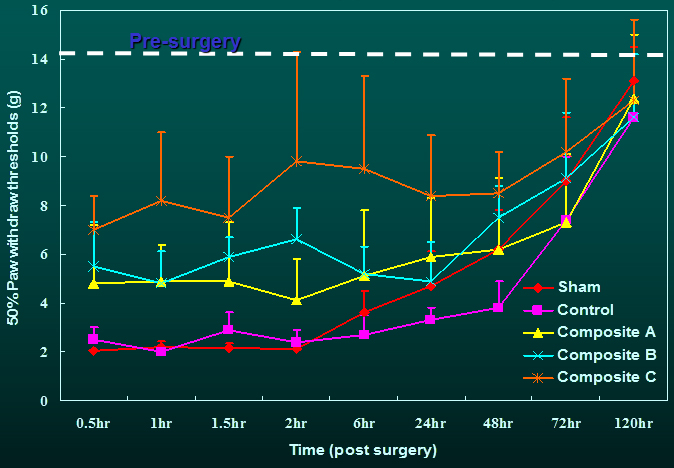
The tactile allodynia
resulting from incision was significantly attenuated in rats receiving
drug-containing composites compared with the control and sham groups for the
duration during which natural healing had not yet taken place. The
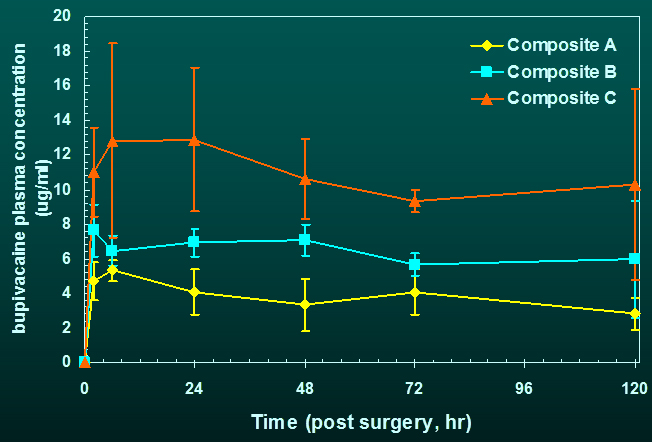
concentration of drug (bupivacaine) in blood is dose dependent and maintained
stable up to 120 h post-surgery, the longest time point measured.
These in vivo studies
show that polymer-xerogel composite materials with controlled release
properties represent a promising class of controlled release materials for pain
management.
© Pharmaceutical Research, 33:729-738, 2016