At the Center for Bioactive Materials and Tissue Engineering research has focused both on fundamental aspects of materials for use in medicine, as well as seeing biomaterials concepts to clinical use.
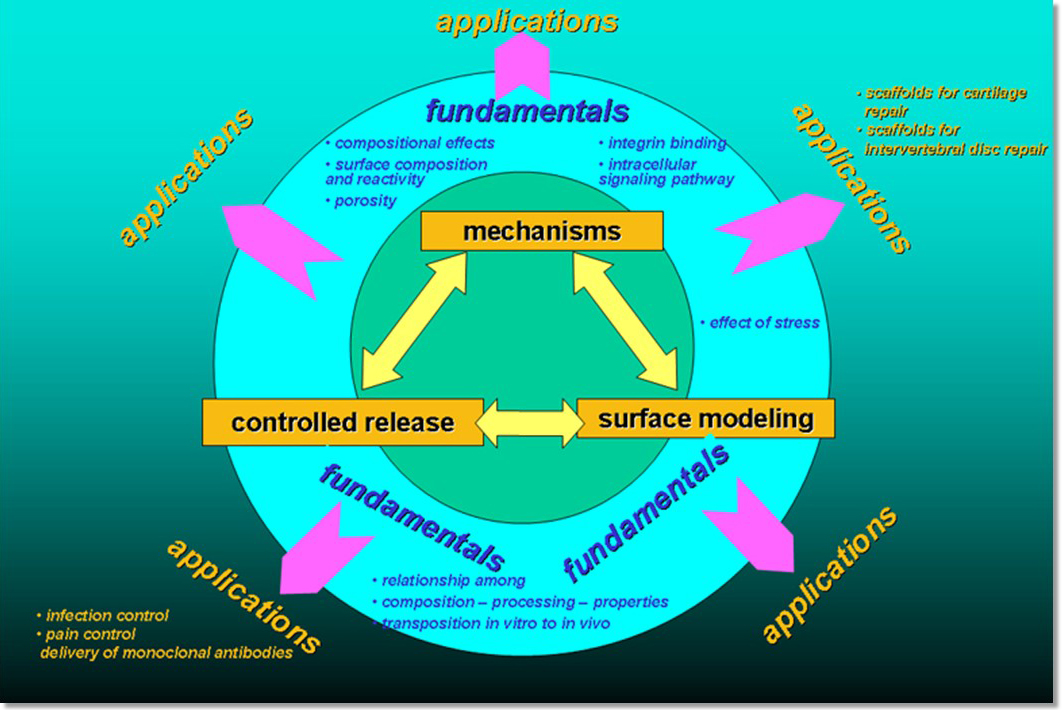
Since the late 1980's when the challenge of in vitro synthesis of bone tissue was first addressed, the investigation of mechanistic effects of materials on cellular functions, specifically cell attachment, proliferation, differentiation and extracellular matrix formation, was at the core of the Center's program. Other studies have focused on the combined effects of microgravity and substrate material on cellular functions; the use of self-assembled monolayer chemistry to create highly controlled surfaces; and, studying the effect of calcification of such surfaces on cell function.
Many PhD students and postdoctoral fellows have come through his program and have become leaders of the next generation. By way of illustration, trainees are Professors at the University of California at Berkeley, the University of Michigan, Columbia University, Georgia Institute of Technology, Drexel University, Kyushu University, Japan, and the K.U. Leuven, Belgium.
With grants from the DoD (Department of Defense) and the NIH through Penn’s Center for Translation, current work focuses on controlled release using advanced, biocompatible silica sol gel concepts. With a growing database of fundamental data in place, applications are related to the device, tissue engineering, pharmaceutics and biotechnology fields. A variety of applications is progressing towards the clinic. Studies focus on combining bone tissue infection treatments with the stimulation of bone tissue repair, growth and regeneration. Other programs are related to infection prophylaxis in surgical sites, including MRSA infections. Breakthrough treatments for osteomyelitis, acquired percutaneous pin tract infections and MRSA treatment are also being pursued.