University of Pennsylvania · Department of Bioengineering
Targeted Antibody & Protein-Based Therapeutics
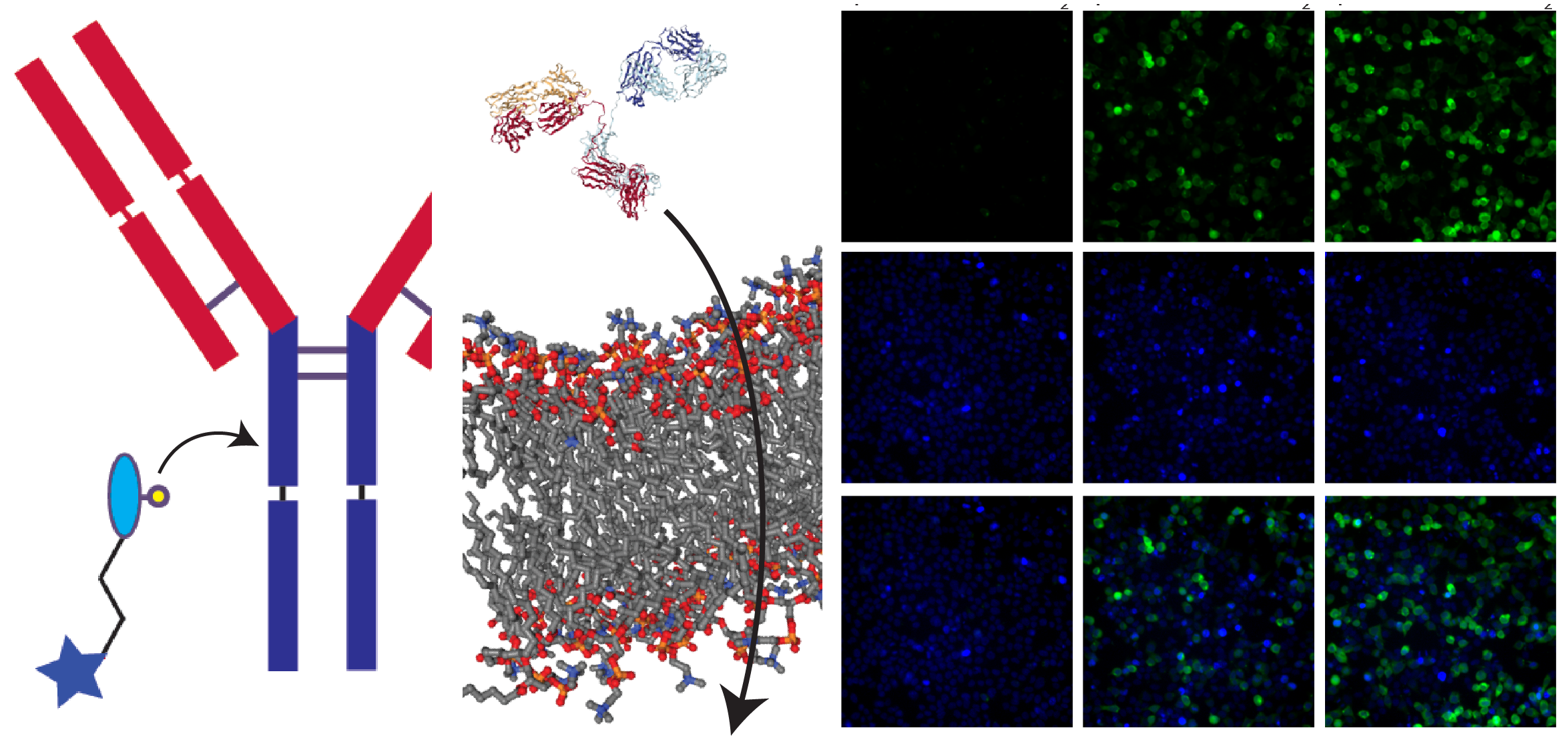 Personalized medicine promises advancements in healthcare by emphasizing early diagnosis of disease, more accurate disease classification and targeted, rather than systemic treatments. These goals are increasingly within reach thanks to the use of immunoassays to detect and classify diseases and the emergence of targeted therapies, many of which rely on nanoplatforms. A central element of many immunoassays and targeted therapies is the use of antibodies or other protein-based targeting ligands. In order to utilize targeting ligands, they frequently need to be conjugated to solid supports, as in the case of immunoassays, nanoparticles, as is increasingly the case with therapeutic or diagnostic payloads, or other functional moieities (e.g. proteins, drugs, etc.). While ideal conjugation methods should possess several features, including biocompatibility, high efficiency, site-specificity, and broad applicability, most existing conjugation methods unfortunately do not meet these requirements. To overcome this challenge, we recently created a suite of bioconjugation techniques that allow for the site-specific and efficient (~100%) attachment of targeting ligands to solid supports, nanoparticles, drugs (e.g. antibody-drug conjugates), or additional targeting ligands (e.g. bispecific antibodies). Our approaches are simple, rapid, scalable and can be used with any targeting ligands ranging in size from a peptide to a full-length antibody. Our lab is now utilizing these tools to create next-generation biologics, including tools for intracellular antibody delivery, T cell redirecting antibodies, and site-specific antibody-drug conjugates. Below are several representative publications:
Personalized medicine promises advancements in healthcare by emphasizing early diagnosis of disease, more accurate disease classification and targeted, rather than systemic treatments. These goals are increasingly within reach thanks to the use of immunoassays to detect and classify diseases and the emergence of targeted therapies, many of which rely on nanoplatforms. A central element of many immunoassays and targeted therapies is the use of antibodies or other protein-based targeting ligands. In order to utilize targeting ligands, they frequently need to be conjugated to solid supports, as in the case of immunoassays, nanoparticles, as is increasingly the case with therapeutic or diagnostic payloads, or other functional moieities (e.g. proteins, drugs, etc.). While ideal conjugation methods should possess several features, including biocompatibility, high efficiency, site-specificity, and broad applicability, most existing conjugation methods unfortunately do not meet these requirements. To overcome this challenge, we recently created a suite of bioconjugation techniques that allow for the site-specific and efficient (~100%) attachment of targeting ligands to solid supports, nanoparticles, drugs (e.g. antibody-drug conjugates), or additional targeting ligands (e.g. bispecific antibodies). Our approaches are simple, rapid, scalable and can be used with any targeting ligands ranging in size from a peptide to a full-length antibody. Our lab is now utilizing these tools to create next-generation biologics, including tools for intracellular antibody delivery, T cell redirecting antibodies, and site-specific antibody-drug conjugates. Below are several representative publications:
1) Wang, H.H., Tsourkas, A. (2019) Cytosolic delivery of inhibitory antibodies with cationic lipids. Proceedings of the National Academy of Science, 116(44), 22132-22139. PMCID:PMC6825285.
2) Greineder, C.F., Villa, C.H., Walsh, L.R., Kiseleva, R., Hood, E.D., Khoshnejad, M., Warden-Rothman, R., Tsourkas, A., Muzykantov, V.R. (2018). Site-specific modification of single-chain antibody fragments for bioconjugation and vascular immunotargeting. Bioconjugate Chemistry, 29(1), 56-66.
3) Warden-Rothman, R., Caturegli, I., Popik, V., Tsourkas, A. (2013) Sortase-Tag Expressed Protein Ligation (STEPL): combining protein purification and site-specific bioconjugation into a single step. Analytical Chemistry, 85(22), 11090-11097. PMCID: PMC3843242.
4) Wang*, H.H., Altun*, B., Nwe, K., Tsourkas, A. (2017) Proximity-based sortase-mediated ligation. Angewandte Chemie, 56(19), 5349-5352. (*contributed equally). PMCID:PMC5537000.
5) Hui, J.Z., Tamsen, S., Song, Y., Tsourkas, A. (2015) LASIC: Light activated site-specific conjugation of native IgGs. Bioconjugate Chemistry, 26(8), 1456-1460. PMCID in process.
Therapeutic and Imaging Agents for Intra-Operative Procedures
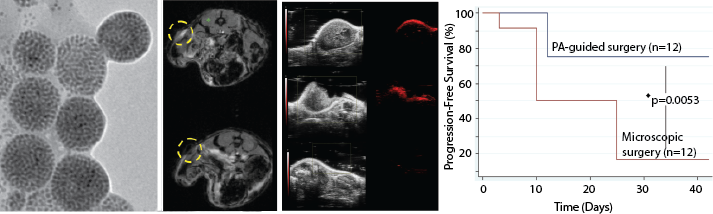 Incomplete tumor resection is a common cause of local tumor recurrence following surgery. Patient outcomes strongly correlate with the extent of surgical resection. To improve removal of residual cancer cells at the time of surgery, we have developed nanoparticle-based agents for both image-guided surgery and photodynamic therapy (PDT). We have shown that in a preclinical surgical resection model in mice, following introduction or our nanoparticles, animals undergoing image-guided surgery demonstrate increased progression-free survival compared to animals undergoing microscopic surgery. Moreover, we have shown that animals that receive PDT demonstrate prolonged survival. These agents can be molecularly targeted and were designed to enable seamless integration into the current standard of care. Below are several representative publications:
Incomplete tumor resection is a common cause of local tumor recurrence following surgery. Patient outcomes strongly correlate with the extent of surgical resection. To improve removal of residual cancer cells at the time of surgery, we have developed nanoparticle-based agents for both image-guided surgery and photodynamic therapy (PDT). We have shown that in a preclinical surgical resection model in mice, following introduction or our nanoparticles, animals undergoing image-guided surgery demonstrate increased progression-free survival compared to animals undergoing microscopic surgery. Moreover, we have shown that animals that receive PDT demonstrate prolonged survival. These agents can be molecularly targeted and were designed to enable seamless integration into the current standard of care. Below are several representative publications:
1) Higbee-Dempsey, E., Amirshaghaghi, A., Case, M.J., Miller, J., Busch, T.M., Tsourkas, A. (2019) Indocyanine green coated gold nanoclusters for photoacoustic imaging and photothermal therapy. Advanced Therapeutics. 2(9), 1900088. PMC in process.
2) Amirshaghaghi, A., Altun, B., Nwe, K., Yan, L., Stein, J.M., Cheng, Z., Tsourkas, A. (2018) Site-specific labeling of cyanine and porphyrin dye-stabilized nanoemulsions with affibodies for cellular targeting. Journal of the American Chemical Society, 140(42), 13550-13553. PMCID:PMC6465177.
3) Yan, L., Amirshaghaghi, A., Huang, D. Miller, J., Stein, J.M., Busch, T.M., Cheng, Z.*, Tsourkas, A.* (2018) Protoporphyrin IX (PpIX)-coated superparamagnetic iron oxide nanoparticle (SPION) nanoclusers for magnetic resonance imaging and photodynamic therapy. Advanced Functional Materials. 28(16), 1707030. PMCID:PMC5997278.
4) Thawani, J.P., Amirshaghaghi, A., Yan, L., Stein, J., Liu, J., Tsourkas, A. (2017) Photoacoustic-guided surgery with indocyanine green-coated superparamagnetic iron oxide nanoparticle clusters extends progression-free survival in a pre-clinical mouse tumor model. Small. 13(37). PMCID:PMC5884067.
Multifunctional Nanoparticles for Cancer Therapy and Imaging
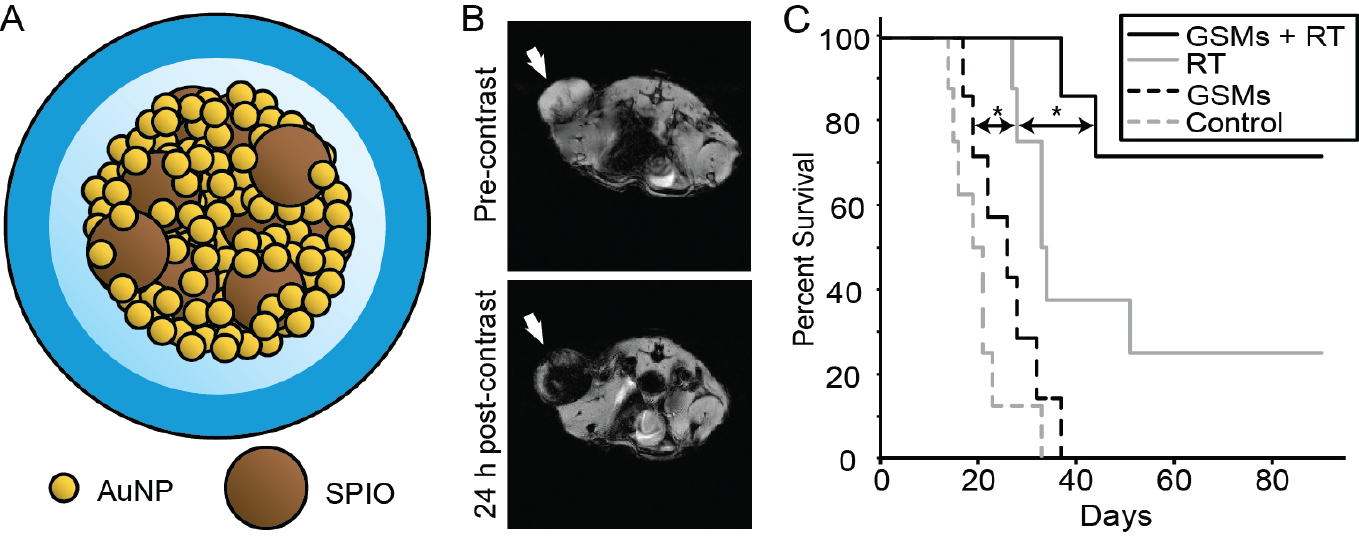 Despite substantial advances in the treatment of many solid malignancies, most chemotherapeies carry a substantial risk of systemic toxicity. This is largely due to their poor pharmacokinetic profiles and broad mechanisms of action. Nanoparticle-based drug delivery systems offer an opportunity to improve the biodistribution, extend the circulation time, and increase the therapeutic index of anti-cancer drugs. Moreover, nanoparticles can also be engineered to include additional functionality such as contrast-enhancement, targeting, magnetic properties, and x-ray attenuating/radiosensitizing properties. This opens the door for new opportunities in cancer treatment; however, with new opportunities comes new challenges, such as reduced tumor penetration, incomplete excretion of nanomaterials, and complex synthesis/poor scalability. We are interested in developing highly efficacious multifunctional nanoparticles, with high tumor accumulation, penetration, and specificity, while keeping scalability, modularity, cost, biodegradability and safety in mind.
Despite substantial advances in the treatment of many solid malignancies, most chemotherapeies carry a substantial risk of systemic toxicity. This is largely due to their poor pharmacokinetic profiles and broad mechanisms of action. Nanoparticle-based drug delivery systems offer an opportunity to improve the biodistribution, extend the circulation time, and increase the therapeutic index of anti-cancer drugs. Moreover, nanoparticles can also be engineered to include additional functionality such as contrast-enhancement, targeting, magnetic properties, and x-ray attenuating/radiosensitizing properties. This opens the door for new opportunities in cancer treatment; however, with new opportunities comes new challenges, such as reduced tumor penetration, incomplete excretion of nanomaterials, and complex synthesis/poor scalability. We are interested in developing highly efficacious multifunctional nanoparticles, with high tumor accumulation, penetration, and specificity, while keeping scalability, modularity, cost, biodegradability and safety in mind.
1) Liu, J., Lan, Z., Ferrari, C., Stein, J., Higbee-Dempsey, E., Yan, L., Cheng, Z., Issadore, D.*, Tsourkas, A.* (2020) Use of oppositely-polarized external magnets to improve the accumulation and penetration of magnetic nanocarriers into solid tumors. ACS Nano. 14(1), 142-152. PMCID:PMC7002255. (*co-corresponding authors)
2) Higbee-Dempsey, E., Amirshaghaghi, A., Matthew, C., Bouché, M., Kim, J., Cormode, D., Tsourkas, A. (2020) Biodegradable gold nanoclusters with improved excretion due to pH-triggered hydrophobic-to-hydrophilic transition. Journal of the American Chemical Society, 142(17), 7783-7794. PMCID:PMC7238296.
3) McQuade*, C., Al Zaki*, A., Desai, Y., Vido, M., Sakhuja, T., Cheng, Z., Hickey, R., Joh, D., Park, S-J, Kao, G.D., Dorsey, J.F., Tsourkas, A. (2015). A multi-functional nanoplatform for imaging, radiotherapy, and the prediction of therapeutic response. Small, 11(7), 834-43 (*contributed equally). PMCID: PMC4329028
4) Al Zaki, A., Joh, D., Cheng, Z., de Barros, A.L., Kao, G.D., Dorsey, J.F., Tsourkas, A. (2014) Gold-loaded polymeric micelles for computed tomography–guided radiation therapy treatment and radiosensitization. ACS Nano, 8(1), 104-112. PMCID: PMC3906892.
Targeted Magnetic Resonance Imaging Contrast Agents
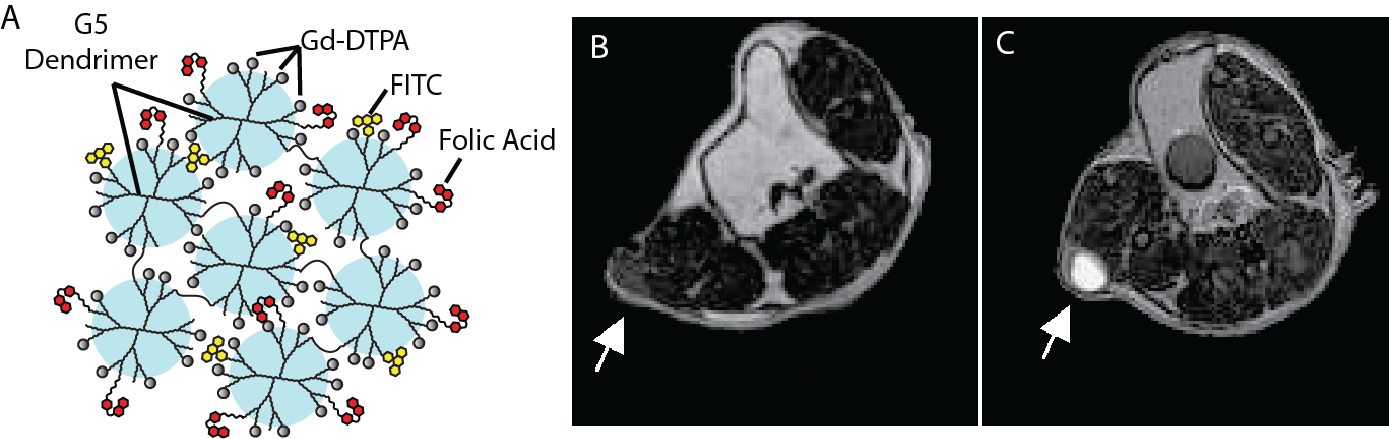 The non-invasive imaging of cancer biomarkers in living subjects could provide a powerful technique for locating metastatic disease, staging tumors, evaluating the availability of therapeutic targets, and monitoring the efficacy of treatment. Magnetic resonance (MR) imaging is a particularly attractive platform for such molecular imaging applications due to its ability to acquire high-resolution anatomical images in conjunction with measures of biomarker expression. However, a major obstacle faced by MR is overcoming the relatively low sensitivity of targeted MR contrast agents. In general, the number of cell receptors at a disease site is too low to recruit enough MR contrast agents to generate sufficient contrast. Therefore, there remains a need to develop new imaging agents capable of generating higher contrast and/or novel amplification strategies that will result in improved targeting. Our lab is pursuing both of these avenues. Specifically, we are developing new formulations of both iron oxide- and gadolinium-based nanoparticles, to improve the contrast-enhancing capabilities per nanoparticle. In parallel, we are developing new amplification schemes that allow for an improvement in the accumulation of contrast agents at the target site. Below are several representative publications:
The non-invasive imaging of cancer biomarkers in living subjects could provide a powerful technique for locating metastatic disease, staging tumors, evaluating the availability of therapeutic targets, and monitoring the efficacy of treatment. Magnetic resonance (MR) imaging is a particularly attractive platform for such molecular imaging applications due to its ability to acquire high-resolution anatomical images in conjunction with measures of biomarker expression. However, a major obstacle faced by MR is overcoming the relatively low sensitivity of targeted MR contrast agents. In general, the number of cell receptors at a disease site is too low to recruit enough MR contrast agents to generate sufficient contrast. Therefore, there remains a need to develop new imaging agents capable of generating higher contrast and/or novel amplification strategies that will result in improved targeting. Our lab is pursuing both of these avenues. Specifically, we are developing new formulations of both iron oxide- and gadolinium-based nanoparticles, to improve the contrast-enhancing capabilities per nanoparticle. In parallel, we are developing new amplification schemes that allow for an improvement in the accumulation of contrast agents at the target site. Below are several representative publications:
1) Cheng, Z., Thorek, D.L.J, Tsourkas, A. (2009) Porous polymersomes with encapsulated Gd-labeled dendrimers as highly efficient MRI contrast agents. Advanced Functional Materials, 19(23), 3753-3759. PMCID: PMC3536029
2) Cheng, Z., Thorek, D.L.J., Tsourkas, A. (2010) Gd-conjugated dendrimer nanoclusters as a tumor targeted T1 magnetic resonance imaging contrast agent. Angewandte Chemie International Edition, 49(2), 346-350. PMCID: PMC2862691
3) Huang, C-H., Nwe, K., Al Zaki, A., Brechbiel, M. Tsourkas, A. (2012) Biodegradable polydisulfide dendrimer nanoclusters as MRI contrast agents. ACS Nano, 6(11), 9416-9424. PMCID: PMC3508381.
4) Yan, L., Higbee, E., Liu, R., Tsourkas, A., Cheng, Z. (2016). A simple method for the synthesis of porous polymeric vesicles and their application as MR contrast agent. Journal of Materials Chemistry B, 3(48), 9277-9284. PMCID:PMC4675335.

