
Metallo-Organic Synthesis: Eric Meggers, Ph.D.
Most of the metal complexes are very rigid and it can therefore
be predicted with high confidence in what conformation they bind
to the active site. Thus, these metal complexes can be considered
imprints of the active site and are therefore useful starting points
for cheminformatics and the design of related purely organic compounds.
The Meggers Lab has recently demonstrated that properly designed
ruthenium compounds can enter mammalian cells and inhibit the protein
kinase GSK-3 without displaying cytotoxicity even at higher micromolar
concentrations. Furthermore, the comparison of the potency in cell-based
assays surpasses significantly the activity of know organic GSK-3
inhibitors (kenpaullone, 6-bromoindirubin-3’-oxime). |
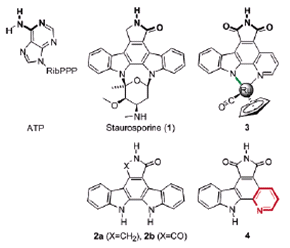 Metallo-organic compounds have Lipinski properties that are entirely
dependent upon the coordinated ligands. The metallo-chemical bond
is as strong as a covalent bond; they do not "fall" apart.
These compounds are designed to be hydrophobic and can pass through
cellular membranes. The rigidity of metallo-organics is potentially
the source of their amazing specificity. These ruthenium coordinated
compounds behave like organic compounds in every respect but they
open new opportunities for accessing rigid globular structures in
an economical fashion. The metal center is not involved in any interactions
with the environment. Space filling models demonstrate that the
metal is not accessible and completely buried in the center of the
molecule. In addition, rapid ligand scanning around a metal center
can be used as a drug discovery tool. It allows the Penn Center
for Molecular Discovery to quickly access unexplored chemical space
and to rapidly identify new biological active and unique structures.
Metallo-organic compounds have Lipinski properties that are entirely
dependent upon the coordinated ligands. The metallo-chemical bond
is as strong as a covalent bond; they do not "fall" apart.
These compounds are designed to be hydrophobic and can pass through
cellular membranes. The rigidity of metallo-organics is potentially
the source of their amazing specificity. These ruthenium coordinated
compounds behave like organic compounds in every respect but they
open new opportunities for accessing rigid globular structures in
an economical fashion. The metal center is not involved in any interactions
with the environment. Space filling models demonstrate that the
metal is not accessible and completely buried in the center of the
molecule. In addition, rapid ligand scanning around a metal center
can be used as a drug discovery tool. It allows the Penn Center
for Molecular Discovery to quickly access unexplored chemical space
and to rapidly identify new biological active and unique structures.