Mechanobiology
Fluid mechanical forces generated by blood flow alter the metabolism of endothelium that line the blood vessel. In 1989, Scott Diamond published in Science an observation that fluid shear stress, instead of a biochemical signal, could control the genetic program of a living cell. The Diamond laboratory proceeded to identify the molecular mechanisms of cellular adaptation and dysfunction in mechanical environments. Mechanical deformations cause endothelial cell calcium mobilization (a regulator of metabolism) and that fluid shear increases endothelial c-fos protein (a regulator of gene transcription). Also, the production of the two most potent vasodilators, nitric oxide (NO) and prostacyclin, is highly coupled during mechanical force induction. In fact, high levels of shear stress shut down gene expression of the potent vasoconstrictor, endothelin, while the vasodilatory genes, eNOS and CNP, are upregulated. Finally, Diamond solved a 150-year old paradox in cardiovascular fluid dynamics called “poststenotic dilatation” where arteries hyperdilate downstream of a narrowing. Diamond discovered that NO is the mediator of this hydrodynamic maladaptation.
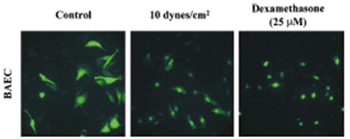
Currently, it remains unknown by what mechanisms exercise and gender lead to protection of the blood vessels. Our lab has discovered (Circulation Res., 2003) that fluid mechanical forces can actually act like the anti-inflammatory steroids(dexamethasone). Fluid shear stress activates the glucocorticoid receptor and turns on genes that contain glucocorticoid response elements in their promoter.
Publications
| 1. |
1989 |
Diamond SL, Eskin SG, McIntire LV. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science 1989; 243 (4897): 1483-1485  |
| 2. |
|
Sharefkin JB, Diamond SL, Dieffenbach CW, Eskin SG. Effects of sustained laminar shear stress on tissue plasminogen activator levels. Cardiovascular Science and Technology 1989; : 81 |
| 3. |
1990 |
Diamond SL, Sharefkin JB, Dieffenbach C, Frasier-Scott K, McIntire LV, Eskin SG. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. J Cell Physiol 1990; 143 (2): 364-371  |
| 4. |
1991 |
Diamond SL, Sharefkin JB, Dieffenbach CW, Eskin SG, McIntire LV. Regulation of Endothelial Cell Gene Expression by Hemodynamic Forces: Implications for Intimal Hyperplasia and Graft Patency. Technologies in Vascular Surgery 1991; : 24-Dec  |
| 5. |
|
Nollert MU, Diamond SL, McIntire LV. Hydrodynamic shear stress and mass transport modulation of endothelial cell metabolism. Biotechnol Bioeng 1991; 38 (6): 588-602  |
| 6. |
|
Sharefkin JB, Diamond SL, Eskin SG, McIntire LV, Dieffenbach CW. Fluid flow decreases preproendothelin mRNA levels and suppresses endothelin-1 peptide release in cultured human endothelial cells. J Vasc Surg 1991; 14 (1): 1-9  |
| 7. |
1993 |
Ranjan V, Diamond SL. Fluid shear stress induces synthesis and nuclear localization of c-fos in cultured human endothelial cells. Biochem Biophys Res Commun 1993; 196 (1): 79-84  |
| 8. |
|
Sigurdson WJ, Sachs F, Diamond SL. Mechanical perturbation of cultured human endothelial cells causes rapid increases of intracellular calcium. Am J Physiol 1993; 264 (6 Pt 2): H1745-1752  |
| 9. |
|
Yamaguchi M, Diamond S, Watanabe H, Gallati H, Baur W, Sharefkin JB. Heparin and dibutyryl cAMP modulate gene expression in stimulated human saphenous vein smooth muscle cells. In Vitro Cell Dev Biol Anim 1993; 29A (11): 867-872  |
| 10. |
1995 |
Ranjan V, Xiao ZS, Diamond SL. Constitutive Nos Expression in Cultured Endothelial-Cells Is Elevated by Fluid Shear-Stress. American Journal of Physiology-Heart and Circulatory Physiology 1995; 38 (2): H550-H555  |
| 11. |
1997 |
Wang W, Diamond SL. Does elevated nitric oxide production enhance the release of prostacyclin from shear stressed aortic endothelial cells?. Biochem Biophys Res Commun 1997; 233 (3): 748-751  |
| 12. |
|
Xiao Z, Zhang Z, Ranjan V, Diamond SL. Shear stress induction of the endothelial nitric oxide synthase gene is calcium-dependent but not calcium-activated. J Cell Physiol 1997; 171 (2): 205-211  |
| 13. |
1999 |
Zhang Z, Xiao Z, Diamond SL. Shear stress induction of C-type natriuretic peptide (CNP) in endothelial cells is independent of NO autocrine signaling. Ann Biomed Eng 1999; 27 (4): 419-426  |
| 14. |
2004 |
Ji JY, Diamond SL. Exogenous nitric oxide activates the endothelial glucocorticoid receptor. Biochem Biophys Res Commun 2004; 318 (1): 192-197  |
| 15. |
2008 |
Ji JY, Jing H, Diamond SL. Hemodynamic regulation of inflammation at the endothelial-neutrophil interface. Ann Biomed Eng 2008; 36 (4): 586-595  |
| 16. |
2009 |
Pagano N, Wong EY, Breiding T, Liu H, Wilbuer A, Bregman H, Shen Q, Diamond SL, Meggers E. From imide to lactam metallo-pyridocarbazoles: distinct scaffolds for the design of selective protein kinase inhibitors. J Org Chem 2009; 74 (23): 8997-9009  |
|





